PSC Market Report; 325 Pages
Recently Released – May 2022
Since the discovery of induced pluripotent stem cell (iPSC) technology 15 years ago, significant progress has been made in stem cell biology and regenerative medicine. New pathological mechanisms have been identified, new drugs identified by iPSC screens are in the pipeline, and the first clinical trials employing human iPSC-derived cell types have been initiated.
iPSCs can be used to explore the causes of disease onset and progression, create and test new drugs and therapies, and potentially, treat previously incurable diseases. The somatic cells used for reprogramming include skin cells and blood cells, and to a lesser degree, other cell types such hair follicles, cord blood and urine.
iPS Cell Commercialization
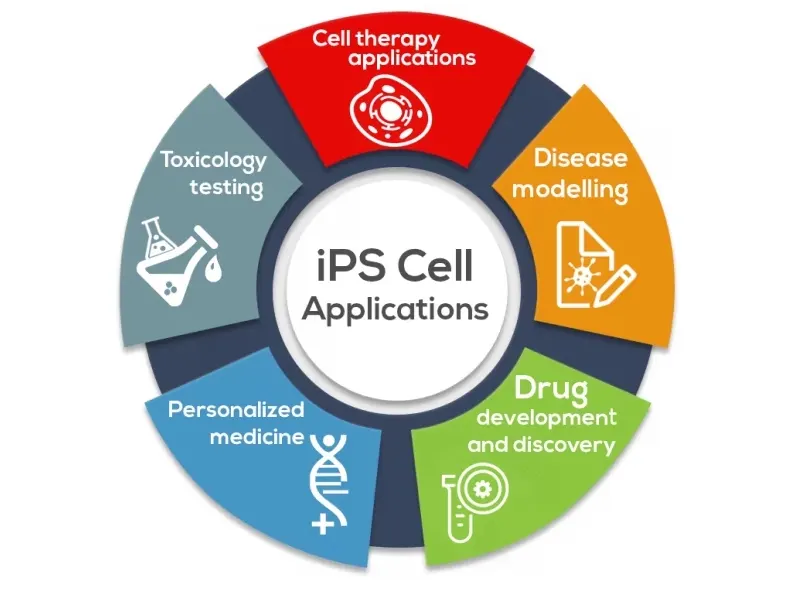
Today, methods of commercializing iPSCs include:
- Cell Therapy: iPSCs are being explored in a diverse range of cell therapy applications for the purpose of reversing injury or disease.
- Disease Modelling: By generating iPSCs from patients with disorders of interest and differentiating them into disease-specific cells, iPSCs can effectively create disease models “in a dish.”
- Drug Development and Discovery: iPSCs have the potential to transform drug discovery by providing physiologically relevant cells for compound identification, target validation, compound screening, and tool discovery.
- Personalized Medicine: The use of techniques such as CRISPR enable precise, directed creation of knock-outs and knock-ins (including single base changes) in many cell types. Pairing iPSCs with genome editing technologies is adding a new dimension to personalized medicine.
- Toxicology Testing: iPSCs can be used for toxicology screening, which is the use of stem cells or their derivatives (tissue-specific cells) to assess the safety of compounds or drugs within living cells.
Other applications of iPSCs include their use as research products, as well as their integration into 3D bioprinting, tissue engineering, and clean meat production. Technology allowing for the mass-production and differentiation of iPSCs in industrial-scale bioreactors is also advancing at breakneck speed.

The Era of iPSCs
In recent years, iPSC-derived cells have increasingly been used to within preclinical testing and early stage-stage clinical trials. The first clinical trial using iPSCs started in 2008, and today, that number has surpassed 100 worldwide. Most of the current clinical trials do not involve the transplant of iPSCs into humans, but rather, the creation and evaluation of iPSC lines for clinical purposes. Within these trials, iPSC lines are created from specific patient populations to determine if these cell lines could be a good model for a disease of interest.
The therapeutic applications of induced pluripotent stem cells (iPSCs) have also surged in recent years. Since the discovery of iPSCs in 2006, it took only seven years for the first iPSC-derived cell product to be transplanted into a human patient in 2013. From 2013 to present, several clinical trials and physician-led studies employing human iPSC-derived cell types have been initiated.
2013 was a landmark year because it saw the first cellular therapy involving the transplant of iPSCs into humans initiated at the RIKEN Center in Kobe, Japan. Led by Dr. Masayo Takahashi, it investigated the safety of iPSC-derived cell sheets in patients with macular degeneration.
In another world first, Cynata Therapeutics received approval in 2016 to launch the first formal clinical trial of an allogeneic iPSC-derived cell product (CYP-001) for the treatment of GvHD. CYP-001 is a iPSC-derived MSC product. In this historic trial, CYP-001 met its clinical endpoints and produced positive safety and efficacy data for the treatment of steroid-resistant acute GvHD.
Given this early success, Cynata is advancing its iPSC-derived MSCs into Phase 2 trials for the severe complications associated with COVID-19, as well as GvHD and critical limb ischemia (CLI). It is also undertaking an impressive Phase 3 trial that will utilize Cynata’s iPSC-derived MSC product, CYP-004, in 440 patients with osteoarthritis (OA). This trial represents the world’s first Phase 3 clinical trial involving an iPSC-derived cell therapeutic product and the largest one ever completed.
Not surprisingly, the Japanese behemoth FUJIFILM has been involved with the co-development and commercialization of Cynata’s iPSC-derived MSCs through its 9% ownership stake in the company. Headquartered in Tokyo, Fujifilm is one of the largest players in regenerative medicine field. It has pursued a broad base in regenerative medicine across multiple therapeutic areas through its acquisition of Cellular Dynamics International (CDI) and Japan Tissue Engineering Co. Ltd. (J-Tec). The Japanese company Healios K.K. is also preparing, in collaboration with Sumitomo Dainippon Pharma, for a clinical trial using allogeneic iPSC-derived retinal cells to treat age-related macular degeneration (AMD).
Riding the momentum within the CAR-T field, Fate Therapeutics is developing FT819, its off-the-shelf iPSC-derived CAR-T cell product candidate. FT819 is the world’s first CAR T therapy derived from a clonal master iPSC line and is engineered with several novel features designed to improve the safety and efficacy of CAR T-cell therapy. Notably, the use of a clonal master iPSC line as the starting cell source could enable CAR-T cells to be mass produced and delivered off-the-shelf at an industrial scale.
Other companies and organizations with iPSC-derived cell therapeutics under development worldwide include:
- Allele Biotechnology and Pharmaceuticals is developing a diabetes drug created from iPSC-derived pancreatic beta cells.
- Aspen Neuroscience is combining stem cell biology and genomics to provide the world’s first autologous induced pluripotent stem cell (iPSC)-derived neuron replacement therapy for Parkinson disease.
- Avery Therapeutics and I Peace, Inc., are collaborating to advance an iPSC-derived cell therapeutic for heart failure. I Peace is generating and supplying GMP-grade iPSCs, while Avery Therapeutics is using them to manufacture its MyCardia™ product.
- Bayer acquired iPSC cell therapy company BlueRock Therapeutics in August 2019. Since May 2021, BlueRock Therapeutics, Fujifilm Cellular Dynamics, and Opsis Therapeutics have had an R&D alliance to develop allogeneic iPSC-derived cell therapies for ocular diseases.
- BlueRock Therapeutics, a subsidiary of Bayer since August 2019, develops iPSC-derived cell therapies to target Parkinson’s disease, heart failure, and ocular diseases.
- Bone Therapeutics has partnered with the U.S. company Implant Therapeutics to develop allogeneic, iPSC-derived MSCs.
- Brooklyn Immuno Therapeutics is developing a set of mesenchymal stem cell (MSC) products, derived from iPSCs, to which it also intends to apply its gene editing technology.
- Century Therapeutics was created in July 2019 by Versant Ventures and Fujifilm to develop iPSC-derived adaptive and innate immune effector cell therapies.
- Citius Pharmaceuticals uses iPSCs from a single-donor dermal fibroblast to create iPSC-derived MSCs (i-MSCs). It has completed the development of an i-MSC Accession Cell Bank (ACB) and is testing and expanding these cells to create an allogeneic cGMP i-MSC Master Cell Bank.
- Cynata Therapeutics manufacturers iPSC-derived MSCs using its proprietary Cymerus™ technology. In partnership with FUJIFILM Corporation, it is clinically testing these cells for the treatment of graft-versus-host disease (GvHD). It is also conducting trials for the treatment of critical limb ischemia (CLI), osteoarthritis (OA), and respiratory failure/distress, including ARDS.
- Cytovia Therapeutics is developing allogeneic “off-the-shelf” gene-edited iNK and CAR (Chimeric Antigen Receptor)-iNK cells derived from iPSCs.
- Edigene, Inc. – Edigene and Neukio Biotherapeutics are developing allogenic iPSC-derived NK cell therapies through an R&D collaboration.
- Editas Medicine (Nasdaq: EDIT), a genome editing company, is developing engineered iPSC-derived natural killer cells (iNKs) for the treatment of cancer.
- Exacis Biotherapeutics is a development-stage immuno-oncology company that is developing NK cells from iPSCs (ExaNK™ cells) engineered using mRNA gene-editing technology to resist rejection by the patient’s immune system.
- Fate Therapeutics is developing iPSC-derived NK and CAR-T cells for the treatment of cancer and immune disorders.
- FUJIFILM Cellular Dynamics, Inc. (FCDI) is investing in a $21M cGMP production facility to support its internal cell therapeutics pipeline, as well as serve as a CDMO for iPS cell products.
- Heartseed Inc. is a Japanese biotech company that is developing iPSC-derived cardiomyocytes (HS-001) for the treatment of heart failure. The company is positioned to initiate a phase 1/2 study of this investigational cell therapy in Japan in the second half of 2021.
- Healios K.K., in collaboration with Sumitomo Dainippon Pharma, is undertaking a clinical trial using allogeneic iPSC-derived retinal cells to treat age-related macular degeneration.
- Hopstem Biotechnology is one of the first iPSC cell therapy companies in China and a market leader in iPSC-derived clinical-grade cell products. In June 2021, it partnered with Neurophth Biotechnology to co-develop an iPSC-derived cell therapy for the treatment of ocular diseases. Hopstem has a proprietary neural differentiation platform, as well as a patented iPSC reprogramming method and GMP manufactory and quality systems.
- Implant Therapeutics is engineering iPSC-MSC cells containing FailSafe™ and induced Allogeneic Cell Tolerance (iACT Stealth Cell™) technologies. These iPSC MSC cells are hypo-immunogenic and can be used as ex-vivo gene therapy vehicles.
- I Peace Inc. and Avery Therapeutics are collaborating to advance an iPSC-derived cell therapeutic for heart failure. I Peace is generating GMP-grade iPSCs, while Avery Therapeutics is using them to manufacture its MyCardia™ product. I Peace is able to mass production clinical-grade iPSC lines simultaneously in a single room using a miniaturized plate and robotic technology, and its facility is equipped with a fully-closed automated iPSC manufacturing system that meets the safety standards of the U.S. FDA and Japanese PMDA.
- Keio University won approval from the the Japanese government in February 2018 for an iPSC trial that involves the treatment of patients with spinal cord injuries (led by Professor Hideyuki Okano).
- Kyoto University Hospital, in partnership with the Center for iPS Cell Research and Application (CiRA), is performing a physician-led study of iPSC-derived dopaminergic progenitors in patients with Parkinson’s disease.
- Neurophth Biotechnology Ltd. is a gene therapy company specializing in AAV-mediated gene therapies for the treatment of ocular diseases. In June 2021, it partnered with Hopstem Biotechnology to develop an iPSC-derived candidate cell product for an agreed upon retinal degenerative disorder.
- Novo Nordisk signed a co-development agreement with Heartseed in mid-2021 that grants it exclusive rights to develop, manufacture, and commercialize HS-001 globally, excluding Japan where Heartseed retained exclusive rights to develop HS-001. HS-001 is an investigational therapy comprised of purified iPSC-derived ventricular cardiomyocytes for the treatment of heart failure.
- Osaka University grafted a sheet of iPS-derived corneal cells into the cornea of a patient with limbal stem cell deficiency, a condition in which corneal stem cells are lost.
- REPROCELL recently launched a “Personal iPS service” in Japan to prepare and store an individual’s iPSCs for the treatment of future illness or injury. Individual’s iPSCs are created from mature cells in their urine or dental pulp, using RNA reprogramming technology. The iPSCs are then stored at two locations in Japan and the U.S.
- RIKEN administered the world’s first iPSC-derived cell therapeutic into a human patient in 2014 when it transplanted an autologous iPSC-RPE cell sheet into a patient with AMD.
- RheinCell Therapeutics GmbH is a developer and manufacturer of GMP-compliant human iPSCs derived from HLA-homozygous, allogeneic umbilical cord blood. In January 2021, the company received GMP certification and Manufacturing Authorization within the EU.
- SCG Cell Therapy Pte Ltd (“SCG”) has acquired the rights to human iPSC technology, from the Agency for Science, Technology and Research (“A*STAR”)’s Accelerate Technologies Pte Ltd (“A*ccelerate”). SCG is using this iPSC technology to to expand its cell therapy product portfolio and develop off-the-shelf NK cell therapies.
- SCM Lifescience, a South Korean stem cell therapy developer, licensed exclusive rights within Korea for the development, approval, production, and sale of a diabetic cell therapy being developed by Allele Biotechnology and Pharmaceuticals. The $750K deal was signed in July 2021.
- Semma Therapeutics, which was acquired by Vertex Pharmaceuticals for $950 million in late 2019, is developing a treatment for Type 1 diabetes. This treatment consists of cells derived from iPSCs that behave like pancreatic cells.
- Shoreline Biosciences is a biotech company that is developing allogeneic “off-the-shelf” natural killer (NK) and macrophage cellular immunotherapies derived from iPSCs for cancer and other serious diseases.
- Stemson Therapeutics has been developing a therapy for hair loss involving generation of de novo hair follicles.
- TreeFrog Therapeutics has a 13,000 sq ft facility in France for the development and scale-up of its cell therapy manufacturing process that leverages human iPSCs. It plans to develop its own iPSC-derived therapies and support co-development programs.
- The U.S. NIH is undertaking the first U.S. clinical trial of an iPSC-derived therapeutic. Its Phase I/IIa clinical trial will involve 12 patients with advanced-stage geographic atrophy of the eye.
- Vita Therapeutics, a Cambrian Biopharma affiliate, is developing iPSC-derived therapeutics, including an autologous, genetically engineered iPSC-derived therapeutic (VTA-100) for limb-girdle muscular dystrophy and a genetically engineered iPSC-derived hypoimmunogenic treatment for muscular dystrophy (VTA-200).
iPS Cell Market Competitors
In addition to the iPSC cell therapy developers, there are an ever-growing number of competitors who are commercializing iPSC-derived products for use across a diverse range of applications. These applications include drug development and discovery, disease modeling, toxicology testing, and personalized medicine, as well as tissue engineering, 3D bioprinting, and clean meat production.
Across the broader iPSC sector, FUJIFILM CDI is one of the largest and most dominant players. Cellular Dynamics International (CDI) was founded in 2004 by Dr. James Thomson at the University of Wisconsin-Madison, who in 2007 derived iPSC lines from human somatic cells for the first time. The feat was accomplished simultaneously by Dr. Shinya Yamanaka’s lab in Japan. FUJIFILM acquired CDI in April 2015 for $307 million. Today, the combined company is the world’s largest manufacturer of human cells created from iPSCs for use in research, drug discovery and regenerative medicine applications.
Another iPSC specialist is ReproCELL, a company that was established as a venture company originating from the University of Tokyo and Kyoto University in 2009. It became the first company worldwide to make iPSC products commercially available when it launched its ReproCardio product, which are human iPSC-derived cardiomyocytes.
Within the European market, the dominant competitors are Evotec, Ncardia, and Axol Bioscience. Headquartered in Hamburg, Germany, Evotec is a drug discovery alliance and development partnership company. It is developing an iPSC platform with the goal to industrialize iPSC-based drug screening as it relates to throughput, reproducibility, and robustness. Today, Evotec’s infrastructure represents one of the largest and most advanced iPSC platforms globally.
Ncardia was formed through the merger of Axiogenesis and Pluriomics in 2017. Its predecessor, Axiogenesis, was founded in 2011 with an initial focus on mouse embryonic stem cell-derived cells and assays. When Yamanaka’s iPSC technology became available, Axiogenesis became the first European company to license it in 2010. Today, the combined company (Ncardia) is a global authority in cardiac and neural applications of human iPSCs.
Founded in 2012, Axol Bioscience is a smaller but noteworthy competitor that specializes in iPSC-derived products. Headquartered in Cambridge, UK, it specializes in human cell culture, providing iPSC-derived cells and iPSC-specific cell culture products.
Of course, the world’s largest research supply companies are also commercializing a plethora of iPSC-related products. Examples of these market leaders include Lonza, BD Biosciences, Thermo Fisher Scientific, Merck, Takara Bio, STEMCELL Technologies, and others.
iPSC Report Features
This report reveals all major market competitors worldwide, including their strategic advantages, novel technologies, and products under development. Its main objective is to describe the current status of iPSC research, biomedical applications, manufacturing technologies, patents, funding events, strategic partnerships, and clinical trials for the development of iPSC-based cellular therapeutics.
Importantly, the report presents a comprehensive market size breakdown for iPSCs by Application, Technology, Cell Type and Geography (North America, Europe, Asia/Pacific, and RoW). It also presents total market size figures and growth rates through 2030.
This global strategic report reveals:
- Market size determinations with segmentation and five-year forecasts
- Clinical trial activity by type, region, phase, and sponsor
- Patent analysis by applicant, type, date and region
- iPSC industry partnerships, alliances, and IPOs
- Emerging trends and future directions
- Competitors composing the global iPSC marketplace
- And much more
This 325-page global strategic report will position you to:
- Capitalize on emerging trends
- Improve internal decision-making
- Reduce company risk
- Approach outside partners and investors
- Outcompete your competition
- Implement an informed and advantageous business strategy
Companies and organizations mentioned in the report include:
– Addgene, Inc.
– Aleph Farms
– Allele Biotechnology and Pharmaceuticals, Inc.
– ALSTEM, INC.
– American Type Culture Collection (ATCC)
– AMS Biotechnology Europe, Ltd. (AMSBIO)
– Applied Biological Materials, Inc. (ABM)
– Applied StemCell (ASC), Inc.
– Aruna Bio, Inc.
– Aspen Neuroscience, Inc.
– Avery Therapeutics
– Axol Bioscience, Ltd
– Bayer
– BD Biosciences
– Beckman Coulter Life Sciences
– BioCat GmbH
– BlueRock Therapeutics (acquired by Bayer)
– Bone Therapeutics
– BrainXell
– Brooklyn Immuno Therapeutics
– Cell Biolabs, Inc.
– Cell Signaling Technology
– Cellaria
– CellGenix GmbH
– Cellular Dynamics International, Inc.
– Cellular Engineering Technologies (CET)
– Censo Biotechnologies, Ltd.
– Century Therapeutics, LLC
– CiRA
– Citius Pharmaceuticals
– Corning, Inc.
– Creative Bioarray
– Cynata Therapeutics Ltd.
– Cytovia Therapeutics
– DefiniGEN
– Exacis Biotherapeutics
– Fate Therapeutics, Inc.
– FUJIFILM Cellular Dynamics, Inc.
– GeneCopoeia, Inc.
– GenTarget, Inc.
– Heartseed, Inc.
– Healios K.K.
– Hopstem Biotechnology
– InvivoGen
– I Peace Inc.
– iPS Portal, Inc.
– iXCells Biotechnologies
– Keio University
– Kyoto University Hospital
– Lonza Group, Ltd.
– Megakaryon Corporation
– Memorial Sloan-Kettering Cancer Center
– Merck/Sigma Aldrich
– Metrion Biosciences, Ltd.
– Miltenyi Biotec B.V. & Co. KG
– Ncardia
– NeuCyte
– Neurophth Biotechnology Ltd.
– Newcells Biotech
– Novo Nordisk
– ONO Pharmaceutical Co., Ltd.
– ORIG3N
– Osaka University
– Oslo University Hospital
– PeproTech
– Phenocell SAS
– Platelet BioGenesis
– Pluricell Biotech
– PromoCell GmbH
– Qiagen
– R&D Systems, Inc.
– ReproCELL
– RheinCell Therapeutics
– RIKEN
– SCG Cell Therapy
– SCM Lifescience
– Semma Therapeutics
– Shoreline Biosciences
– STEMCELL Technologies
– Stemina Biomarker Discovery
– Stemson Therapeutics
– Sumitomo Dainippon Pharma
– Synthego Corp.
– System Biosciences (SBI)
– Takara Bio
– Takeda Pharmaceutical Co., Ltd.
– Tempo Bioscience
– Thermo Fisher Scientific, Inc.
– TreeFrog Therapeutics
– University of California
– U.S. NIH
– Vertex Pharmaceuticals
– VistaGen Therapeutics, Inc.
– Vita Therapeutics
– Waisman Biomanufacturing
– xCell Science, Inc.
– Yashraj Biotechnology, Ltd.
With the competitive nature of this global market, you don’t have the time to do the research. Claim this report to become immediately informed, without sacrificing hours of unnecessary research or missing critical opportunities.
About BioInformant
With an online readership of nearly one million viewers per year, BioInformant is a U.S. market research firm with nearly 15 years of experience. As the first and only market research firm to specialize in the stem cell industry, BioInformant research has been cited by the Wall Street Journal, Xconomy, and Vogue Magazine. Headquartered in Washington, DC, BioInformant is strategically positioned to be near the National Institutes of Health (NIH), the U.S. FDA, the Maryland Biotech Corridor, and policy makers on Capital Hill.
To create this report, BioInformant’s analysts interviewed hundreds of highly regarded iPSC industry leaders, including those those featured here. These KOLs include Kaz Hirao (President & COO of FUJIFILM CDI), Ross Macdonald (CEO of Cynata Therapeutics), Robin Smith (CEO of ORIG3N), Paul Wotton (Board Member of Cynata Therapeutics), and Yutaka Yamaguchi (President of FUJIFILM Irvine Scientific), and others.
source: